Electrochemical energy storage has the advantages of small geographical restrictions, short construction period, and continuous cost reduction. At the same time, it can alleviate the problem of poor stability of renewable energy, and is expected to become the mainstream energy storage technology in the future. Below we introduce two common energy storage batteries:
Lithium-ion batteries dominate electrochemical energy storage market
Lithium-ion energy storage batteries are composed of positive electrodes, negative electrodes, electrolytes and separators. It has the advantages of high energy density, no memory effect, fast charging and discharging, fast response, flexible configuration, and short construction period. It is widely used in wind power photovoltaic and other new energy generation side, grid side, and user side energy storage projects.
Energy density:
The energy density of lithium-ion energy storage batteries refers to the electrical energy that the battery can store per unit mass. The energy density of lithium-ion batteries is high, generally between KW and MW, which is much higher than that of lead-acid batteries. High energy density means that lithium-ion batteries can store more electricity for the same battery quality, thus providing longer use time.
Lifespan:
The lifespan of a lithium-ion energy storage battery is usually measured in terms of cycle life, which is how many charge-discharge cycles the battery can undergo. Lithium-ion batteries have a long life, typically capable of thousands of charge and discharge cycles, compared to lead-acid batteries, which have a lifespan of only a few hundred. At the same time, lithium-ion batteries have a low self-discharge rate, which means that the energy in the battery can be retained for a longer period of time even if it has not been used for a long time.
Charge and discharge efficiency:
The charge and discharge efficiency of lithium-ion energy storage batteries refers to the ratio between the electric energy output by the battery and the electric energy input during the charge and discharge process. The charge and discharge efficiency of lithium-ion batteries is relatively high, usually above 95%, which means that the battery can be charged and discharged quickly in a short time while effectively reducing energy loss.
Safety:
Lithium-ion energy storage batteries require special attention to safety during use, because under improper use or storage conditions, batteries may overheat, explode and other safety issues. In order to improve the safety performance of lithium-ion batteries, battery manufacturers usually adopt a variety of safety protection measures, such as overcharge protection, over-discharge protection, over-current protection, etc.
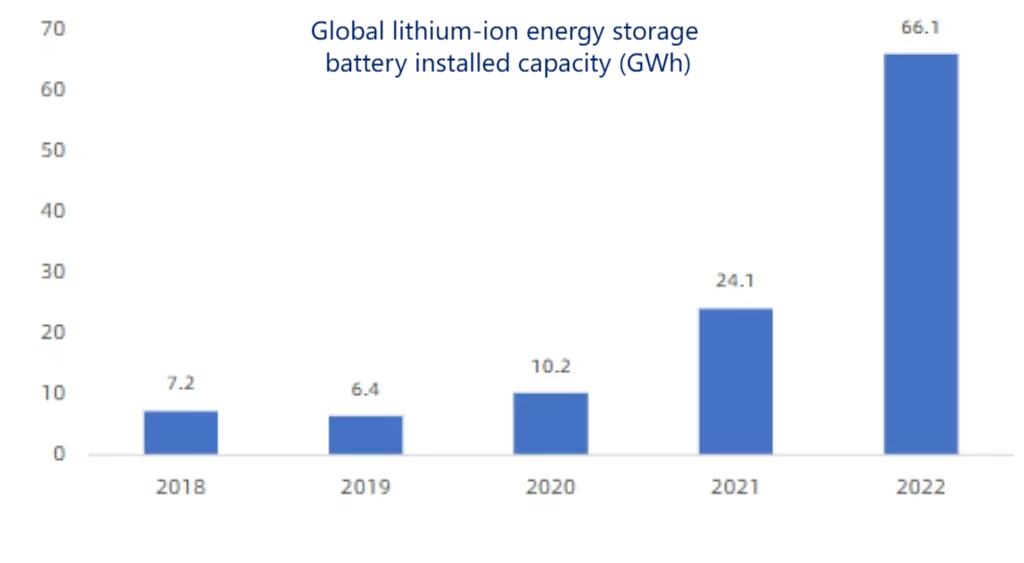
The scale of new installed capacity of lithium-ion energy storage batteries worldwide continues to grow. It will continue to grow from 7.2GWh in 2018 to 66.1GWh in 2022, with an average annual compound growth rate of approximately 74.1%.
Lithium iron phosphate battery is a lithium battery mainly used for energy storage at this stage.
The charging and discharging process of lithium iron phosphate (LiFePO4) battery mainly relies on the migration of lithium ions. Compared with other lithium batteries such as ternary materials, lithium iron phosphate battery technology is relatively mature, and its structure is still relatively stable even at high temperatures. Lithium iron phosphate has It far exceeds the safety and stability of other cathode materials and meets the stringent safety requirements of large-scale energy storage projects.
The energy density of lithium iron phosphate is lower than that of ternary materials, but the comparative advantages brought by high cycle life and low raw material costs are more central to the energy storage field where battery volume requirements are not high. The advantages of safety, long cycle life and low raw material costs give lithium iron phosphate batteries significant competitive advantages.
Lead-acid battery: mature technology, low cost, small scale of energy storage installation
Lead-acid batteries can be divided into two types: lead-acid batteries and lead-carbon batteries.
Lead-acid batteries
Lead-acid batteries have a history of more than 150 years since they were invented by Plante in 1859. The technology is very mature and it is the most widely used chemical power source in the world. Although new batteries such as lithium-ion batteries have been introduced and applied in recent years, lead-acid batteries still rely on their strong high-current discharge performance, stable voltage characteristics, wide temperature range, large single battery capacity, high safety and abundant raw materials and can be used. A series of advantages such as recycling and low price occupy a solid position in most traditional fields and some emerging application fields.
Lead-acid batteries are widely used in automobiles, trains, tractors, motorcycles, electric vehicles, communications, power stations, power transmission, instrumentation, UPS power supplies and aircraft, tanks, ships, radar systems and other fields. With the development of the world’s energy economy and the increasing improvement of people’s living standards, lead-acid batteries have occupied more than 85% of the market share in secondary power supply.
Lead-acid batteries have the advantages of mature technology, low cost, good high-current discharge performance, wide applicable temperature range, high safety, and can be fully recycled. They cannot be replaced by other batteries in the field of automobile starting batteries and electric vehicles.
Lead-carbon batteries
Lead-carbon batteries are a type of capacitive lead-acid battery, a technology that evolved from traditional lead-acid batteries. The biggest difference between lead-carbon batteries for electric energy storage and ordinary lead-acid batteries lies in the negative electrode of the battery. If all the negative active material Pb in an ordinary lead-acid battery is replaced by activated carbon, the ordinary lead-acid battery becomes a hybrid capacitor; if activated carbon is mixed into the negative plate of an ordinary lead-acid battery, it becomes a lead-carbon battery.
Lead-carbon batteries have the characteristics of both lead-acid batteries and capacitors. Because carbon materials are added to the negative electrode of lead-carbon batteries or pure carbon materials are used, the sulfation phenomenon of the negative electrode is alleviated or solved, so lead-carbon batteries have good cycle life and charging performance. Lead-carbon batteries not only take advantage of the instant large-capacity charging of supercapacitors, but also take advantage of the energy advantages of lead-acid batteries.
Recap
Various batteries excel in unique application fields, each offering distinct advantages tailored to specific use cases and actual requirements. Interested in our lead-acid batteries and lithium-ion batteries, please feel free to contact us!